Absorption Vs Adsorption
Absorption Vs Adsorption
Absorption and Adsorption terms seems very similar but they are quiet different. Many of us got confused between them frequently. The main difference between absorption and adsorption is that absorption is the operation in which a fluid(or gaseous mixture) brings in contact with a liquid for the separation of specific gas component into that liquid where as in case of Adsorption, the atoms, ions, or molecules from the Liquid or Gaseous Mixture adhere to the solid surface of the adsorbent. Both processes basically falls under the category of separation techniques. Lets talk about them in detail.
Definition
1. Absorption
- Absorption is an operation in which a gas mixture is contacted with a liquid for preferentially dissolving one or more components of the gas and to provide a solution of them in liquid.
- In ammonia plant for production of synthesis gas carbon dioxide is absorbed in an aqueous solution of an Alkanolamine.
Aricle written By : Pooja Bandagale
2. Adsorption
- Adsorption is selective concentration or retention of one or more components of a mixture on a solid surface. Molecules distribute themselves between two phases, one of which is a solid whilst the other may be a liquid or a gas.
- The substance which gets adsorbed on the surface of solid is called “adsorbate” and the substance on which it is adsorbed is called “adsorbent”.
- For items as diverse as electronic instruments and biscuits, sachets of adsorbent may be included in the packaging in order to keep relative humidity low.
Mechanism
Absorption
- Two film theory: According to this theory, material is transferred in the bulk of the phases by convection and concentration differences are regarded as negligible except in the vicinity of the interface between the phases. On either side of this interface there exists a thin film of fluid through which the transfer is effected solely by molecular diffusion.
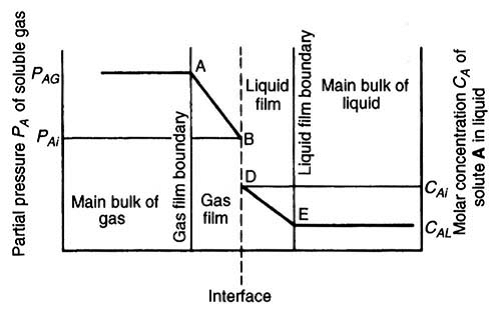
- For a chemical absorption there exist a zone of reaction between A & B which moves away from gas-liquid interface
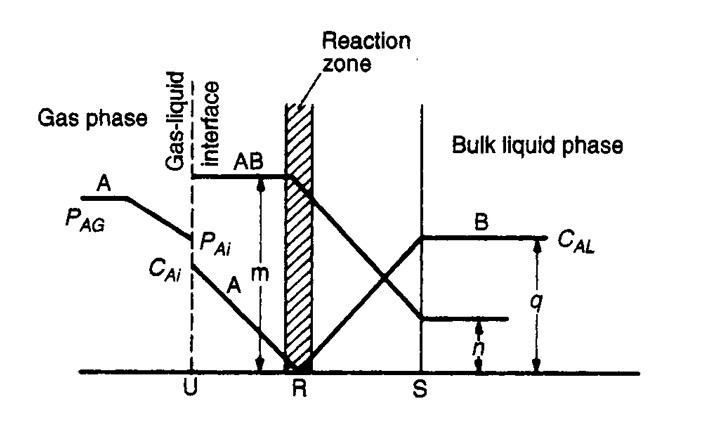
Adsorption
- Unlike absorption, in which solute molecules diffuse from the bulk of a gas film to the bulk of liquid phase, in adsorption molecules diffuses from the bulk of the fluid to the surface of solid adsorbent forming a distinct adsorbed phase.
- It is often convenient to think of adsorption as occurring in three stages.
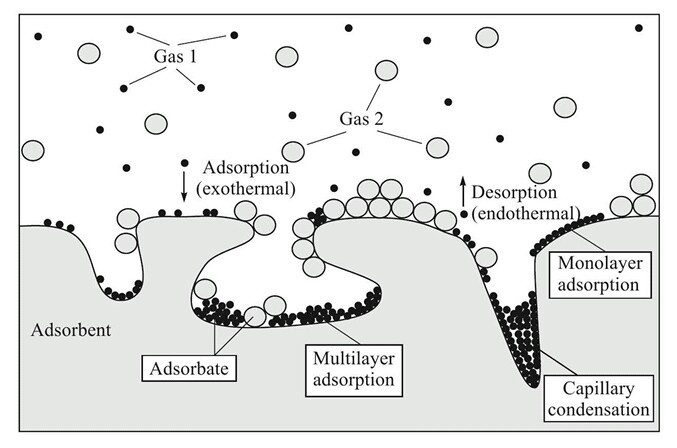
- Firstly, a single layer of molecules build up over the surface of the solid. This monolayer may be chemisorbed and it is associated with change in free energy.
- As the fluid concentration further increases, more layers are formed by physical adsorption and the number of layers formed may be limited by size of pore.
- Finally, for adsorption from gas phase, capillary condensation may occur in which capillary become filled with condensed adsorbate.
Nature
Absorption
- Many absorbers and strippers deal with dilute gas mixtures and liquids, and it is frequently satisfactory in these cases to assume that the operation is isothermal.
- In some absorption processes, especially where a chemical reaction occurs, there is liberation of heat. This generally gives rise to an increase in the temperature of the liquid. This increase in temperature will reduce the solubility of gases in liquid.
Adsorption
- Adsorption is always accompanied by the liberation of heat. For physical adsorption, the amount of heat is similar in magnitude to the heat of condensation. For chemisorptions it is greater and of an order of magnitude associated with chemical reaction.
Effect of temperature and pressure
Absorption
- The solubility of a gas in a liquid decreases with temperature. Thus absorption of a gas favors at lower temperature so that there is a larger driving force for transfer of solute from gas phase to the solvent. Desorption or stripping, on the other hand, is carried out on an elevated temperature so that direction of driving force is reversed.
- As pressure increases absorption power increases.
PA = HA * xA ( Henry’s law)
Adsorption
- Pressure or concentration and temperature are the two most important variables that determine the amount of a solute adsorbed per unit mass of the adsorbent at equilibrium. It is favored at a higher pressure and a lower temperature. Conversely, a lower pressure and higher pressure favors desorption.
Types
Absorption and adsorption processes are divided into two groups in which the process is solely physical and those where a chemical reaction is occurring.
Absorption
- Acetone can be recovered from an acetone-air mixture by passing the gas stream into water in which the acetone dissolves while acetone passes out. In this the process of absorption of gas in the liquid is physical process because chemical reaction having no appreciable effect.
- A chemical reaction occurs when oxides of nitrogen are absorbed in water to give nitric acid. The nature of reaction influences the actual rate of absorption.
Adsorption
- Physical adsorption, or van der Waals adsorption, a readily reversible phenomenon, is the result of intermolecular forces of attraction between molecules of the solid and the substance adsorbed. It can be monolayer or multilayer.
- Chemisorption, or activated adsorption, is the result of chemical interaction between the solid and the adsorbed substance. It is monolayer phenomena.
Applications
Absorption
Absorption of gases in liquid phase is used for
- Purification of gases
- Separation of gases
- Production of useful liquid product
Adsorption
Adsorption from the liquid phase is used to
- Remove organic components from aqueous wastes
- Colored impurities from sugar solutions and vegetable oils
- Water from organic liquids.
Content and image Sources :
[Binay_K._Dutta]_Principles_Of_Mass_Transfer_And_S(b-ok.cc)