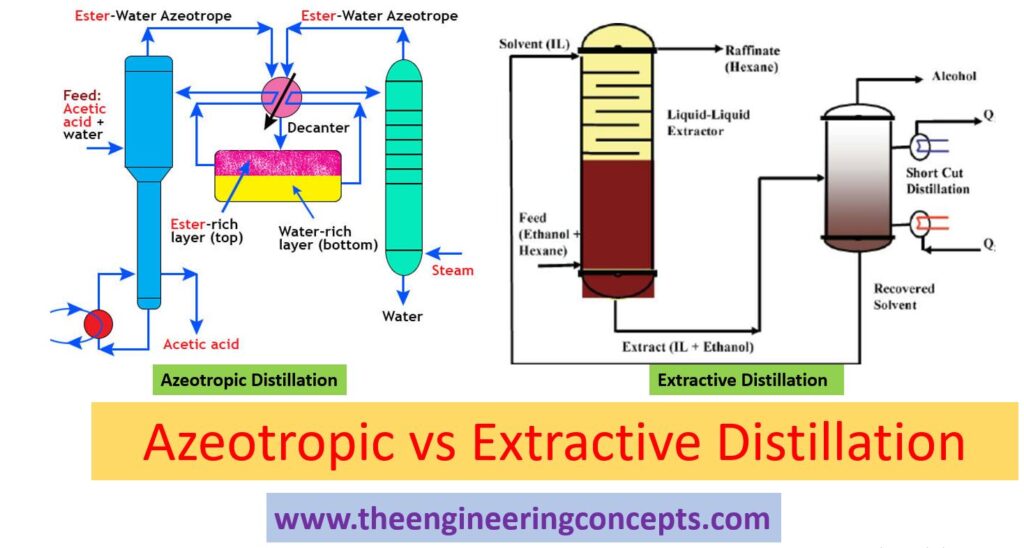
Azeotropic Vs Extractive Distillation
Azeotropic and Extractive distillation are the type of distillation processes that are widely used in the field of engineering and chemistry and pharmaceutical industries and also to improve the product research. On the other hand, distillation is one of the most important processes in separation of components from mixture such as separating gasoline, diesel, kerosene and other hydrocarbon from crude oil. Distillation works on the principle of separation of components based on their boiling points or relative volatility of the components in the mixture of the boiling liquid. In Chemistry, these two distillation techniques are very essential in the separating certain mixtures to form a new product. Besides, these processes have some similarities and differences as well.
Aricle written By : Pallavi Wankhede
Azeotropic Distillation:
- Azeotropic distillation is used to break the azeotropes. Azeotrope is also called constant boiling point mixture; it is a composition of two or more liquids whose proportions can be separated or altered with the help of simple distillation. Azeotropic distillation is a process of adding another component to form a new azeotrope with lower boiling point with immiscible liquid phases.
- Addition of such components gives formation of separate phase. In both the distillation cases, the addition of components to mixture for separation might seem similar.
- It is difficult to purify completely the low boiling azeotropes or volatile components by simple distillation.
- To obtain the pure volatile components, it is necessary to break those low boiling azeotropes. Thus, for separation new approach is implied rather than distillation (not useful for separation in this case). This new approach is nothing but the use of molecular sieves (material having very small pores with uniform size with 2nm or less, which helps to absorb only smaller components and escape components with larger size and high molecular weight). Zeolites, Activated charcoal and silica gel are also used for the formation of such sieves and also used as desiccants.
- For an instance, treatment of 96% ethanol with molecular sieve gives the formation of anhydrous alcohol where sieves absorbed the water from the mixture. While the sieves can be regenerate afterwards with the help of dehydration using vacuum oven.
Figure 1 : VLE for mixture of ethanol and water showing Azeotropic Point
- Process: The design setup for dehydration unit may vary depending on the condition of hydrous ethanol feedstock and the presence of an alcohol distillation plant. The given type of setup (fig 3) is stand-alone drying units for liquid feed, which is used for hydrous ethanol liquid from storage, while hydrous ethanol is vaporized in a small recycle column. The regeneration or purge stream is returned to the recycle column for recovery of ethanol. The energy consumption of the ethanol drying unit is minimized by an optimal design of heat recovery consideration of feedstock and utility conditions. Besides, the molecular sieve dehydration employs an adsorption process using synthetic zeolite (sieve material), a crystalline and highly porous material. The process is based on the principle that zeolite’s affinity (sieve) for water changes at different pressure. The water loading of the zeolite (sieve material) depends on the partial pressure of the water in the feed which can be influenced by altering the pressure.
Extractive distillation:
- Extractive distillation takes place in the presence of miscible component having high boiling point and are non-volatile. It doesn’t form azeotropes with other components in the mixture. This process is used for mixtures with low relative volatility, approximately unity. Mixtures with relative volatility of one or nearly equal are difficult to separate by simple distillation.
- The solvent used for separation is generally non-volatile, having high boiling point and miscible with the mixture and prevents formation of azeotropes.
- This solvent helps to change the relative volatilities of the components in the mixture.
- This action of solvent makes possible the separation of three part mixture by simple distillation. While choosing a separation solvent, it is necessary to consider the cost, quantity, and availability of solvent.
- For an instance, the separation of an azeotropic mixture of benzene and non-aromatics, the separation solvent used is NFM (N-Formylmorpholine). The bottom product consist of a mixture of NFM, a little amount of benzene and non-aromatic components, which can again be separated easily as the NFM (solvent) does not form an azeotrope with other components. While the bottom product can be separated by any methods available. The care must be taken that the solvent should not react chemically with the mixture or does not cause corrosion in the equipment.
- Process: The extractive distillation setup usually consists of two units, extractive distillation column and solvent recovery column (fig 5). The given setup is industrial benzene extraction plant (fig 4, 5). It consists of three main sections, pre-distillation, extractive distillation and solvent regeneration. In pre-distillation section, the feed (mixture of benzene and non-aromatics) is separated to toluene and benzene fractions. Benzene fractions then enter the extractive distillation column and get recovered with the help of separating solvent NFM (N-Formylmorpholine). Where NFM solvent change the vapour pressure and make ease the removal of non-aromatics (paraffin, naphthene) from aromatics during distillation. In extractive distillation column, the overhead product consist of non-aromatics, benzene and solvent in the form of vapours are then fed into solvent recovery column. The solvent recovery column separates the solvent and non-aromatics. While, the bottom product consists of solvent, benzene and a small amount of non-aromatic are then conveyed through the stripper column. The stripper column obtains pure benzene as overhead product with the help of vacuum distillation (solvent regeneration unit). The stripped hot solvent from the bottom of the stripper column is pumped through several heat exchangers to the top of extractive distillation column.
Content Source:
https://en.wikipedia.org/; https://www.scielo.br/;
Image Source:
https://www.epicmodularprocess.com/; https://www.vogelbusch-biocommodities.com/; https://www.researchgate.net;