Gas Chromatography
Abstract : Gas Chromatography
Gas chromatography is one of the most widely used techniques for analyzing hydrocarbon mixtures. Some of the advantages of chromatography are the range of measurement (from ppm levels up to 100 %), the detection of a wide range of components, and the repeatability of the measurements. Chromatography is used in the laboratory, in permanently installed online systems, and in the field with portable systems. No matter the location, style, or brand, all gas chromatographs are composed of the same functional components: the sample handling system, the chromatograph oven, and the controller electronics (refer to below Figure).
This post will cover the principles of sample handling, how chromatograph columns separate the components, why and how multi-port analysis valves are used, the common detector type used in the hydrocarbon applications, and the analysis processing that provides the component concentrations and the other calculated values (such as heating value and specific gravity) through physical reports or interfaces to other devices.
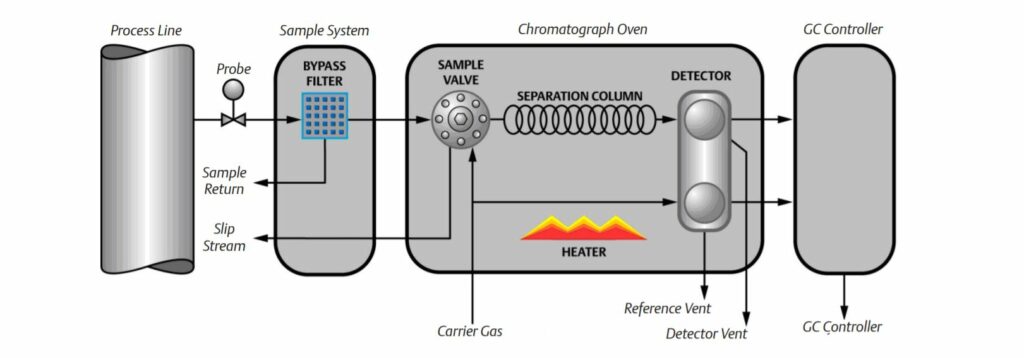
Working Principle of Gas chromatograph
Sample Handling System
The gas chromatograph uses very small diameter gas paths and significant restrictions to flow; therefore, the sample injected to the analyzer must be dry and free of solids. Additionally, the gas chromatograph requires samples to be injected at a pressure significantly different than the source of the sample. The sample handling system should control the sample pressure and remove solid and liquids to ensure the sample presented to the gas chromatograph is clean and dry. The stream selection on multi- stream applications is also a function of the sample handling systems and is usually performed by simple solenoid valves.
Most gas chromatographs require the sample to be controlled typically to between 15 and 30 PSIG (100 and 200 kPaG). In the majority of applications, the sample will be at considerably higher pressures, so the sample pressure must be regulated down to within the acceptable range.
Thompson effect in which the temperature of a gas will decrease as the pressure is reduced. Whereas the amount of cooling is dependent on the composition of the gas, a typical value used.for estimating the cooling of natural gas is seven degrees Fahrenheit per 100 PSI (American Gas Association, n.d.) or 5.6 °C per 1000 kPa. As the temperature of a hydrocarbon gas mixture is reduced, eventually some of the larger hydrocarbon components will begin to drop out of the gas phase into the liquid phase. The temperature at which this begins to occur is the hydrocarbon dew point. If the sample is near the hydrocarbon dew point when it is sampled, the temperature of the gas may drop below this point when the pressure is regulated down, and the composition of the gas will change as the heavy components drop out as liquids.
The goal of the sample handling system is to preserve the composition of the gas, so it is important to consider the detrimental effects that the Joules-Thompson effect may have on the sample.
The use of heated regulators, insertion regulating probes (which use the heat from the flowing gas in the pipeline to maintain the temperature of the regulated sample), and heated sample handling components such as heat-traced tubing is highly recommended to ensure that the temperature of the sample is maintained to at least 30 °F (16.7 °C) above the hydrocarbon dew point (American Petroleum Institute, 2006).
Inline filters are typically used to remove solid particles down to two- microns in size. The larger the volume inside the sample handling system, the longer the lag time between when the sample enters the probe and when it enters the gas chromatograph. To avoid introducing large sample lag times into the system, particulate filters should not have large volumes, so coalescing filters are generally not recommended.
Many sample probes in use today include a particulate filter at the filter tip. These are highly recommended as they stop contamination entering the system; however, users must routinely remove the probe and replace the filters to ensure the sample flow through the filter/probe is sufficient.
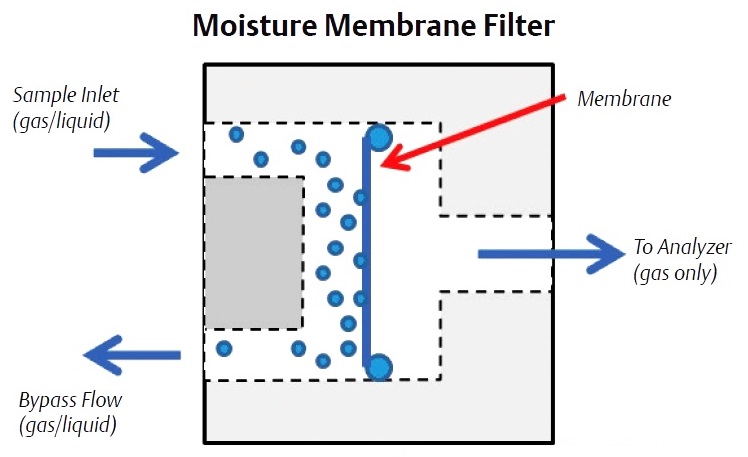
Liquids can be removed using membrane filters that use a specially designed membrane that only permits gas to pass through. The membrane can be located on the probe tip or in a membrane filter/ separator located on the gas chromatograph sample handling system. The typical liquid membrane filter passes the sample gas through the membrane and sweeps the removed liquids away through a bypass flow path. The bypass flow also allows the sample lag time to be reduced as it provides for a faster flow rate from the probe to the analyzer sample handling system (up to 500 cc/min) than the flow rate into the gas chromatograph, which is typically 30 cc/min to 50 cc/min.
Chromatograph Oven
The analytical components of the gas chromatograph (the columns, valves, and detectors) are enclosed in a heated oven compartment.
The performance and response of the chromatograph columns and the detectors are very susceptible to changes in temperature, so the oven is designed to insulate these components from the affects of ambient temperature changes and maintain a very stable temperature internally. A gas chromatograph’s performance is directly correlated to the temperature stability of the columns and detector, so the temperature control is typically better than ± – 0.5 °F (± – 0.3 °C)
The temperature at which the oven is controlled is dependent on the application: the heavier the expected hydrocarbon mixture, the hotter the oven temperature. Natural gas applications have a typical oven temperature setting of around 176 °F (80 °C). The temperature of the oven is typically set in the factory and is rarely adjusted in the field.
Chromatograph Columns
The heart of the gas chromatograph is the chromatograph columns. The columns separate the gas mixture into its individual components using some physical characteristic. In the case of most hydrocarbon applications, “boiling point” columns are used and separate the components by their individual boiling points; however, other applications may use molecular size (molecular sieve columns) or polarity differences to achieve the separation.
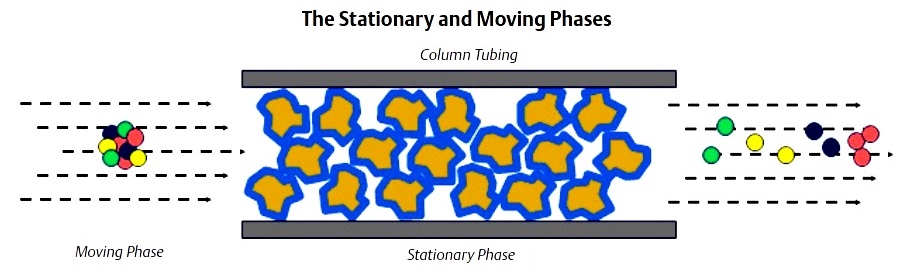
Chromatograph columns are constructed by packing a tube with column packing material. The material is held in place by sintered metal filters at either end of the tube. The packing material consists of very small support material that has a very thin coating of liquid solvent. This is called the stationary phase.
The sample gas is carried through the columns by the carrier gas. The combination of the carrier gas and the sample gas is called the moving phase. The carrier gas is a gas which is not a component of interest (it is not being measured) and acts as a background gas that permits the easy detection of the components being measured.Typically, helium is used for hydrocarbon applications; however, hydrogen, argon, and nitrogen are also used, depending on the application.
As the gas sample moves through the column, components with lower boiling points move more slowly than the components with higher boiling points. The speed at which this separation occurs is dependent on the temperature of the column. The length of the column determines the amount of separation of the components.
Analysis Valves
Theoretically, a single column could separate all of the components of interest in a sample; however, the column would be very long, and the analysis time would be longer than 30 minutes. To accelerate the analysis time to a more practical time, multiple columns are used to split the analysis into smaller and faster column applications. In a typical four-minute C6+ three-column arrangement (refer to below Figure under Analysis Valves topic), column one separates the hexanes and heavier components (the C6+ components) from the pentanes to methane hydrocarbons, nitrogen, and carbon dioxide. Column two then separates normal-pentane, iso-pentane, neo-pentane, normal-butane, and iso-pentane, while allowing the nitrogen, methane, carbon dioxide, and ethane to pass on to and be separated by column three.
The injection of sample into the analyzer (the sample valve) and the switching of the analytical flow paths to enable the use of multiple columns are achieved using specialized analysis valves. Analysis valves come in either six-port or ten-port configurations with very low internal volumes to avoid any dead volumes and to allow precise switching between components as they leave the columns. As the analysis valves are located inside the oven compartment, they are pneumatically operated through three way solenoids mounted outside of the heat-controlled zone of the oven. In some applications, the carrier gas is used as the actuation gas; however, clean and dry air or nitrogen also can be used to reduce expensive carrier gas usage.
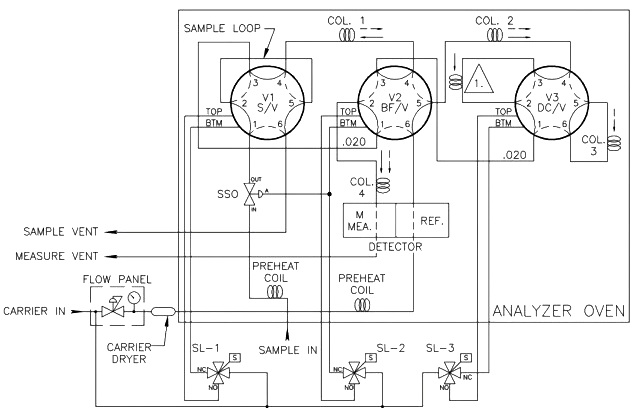
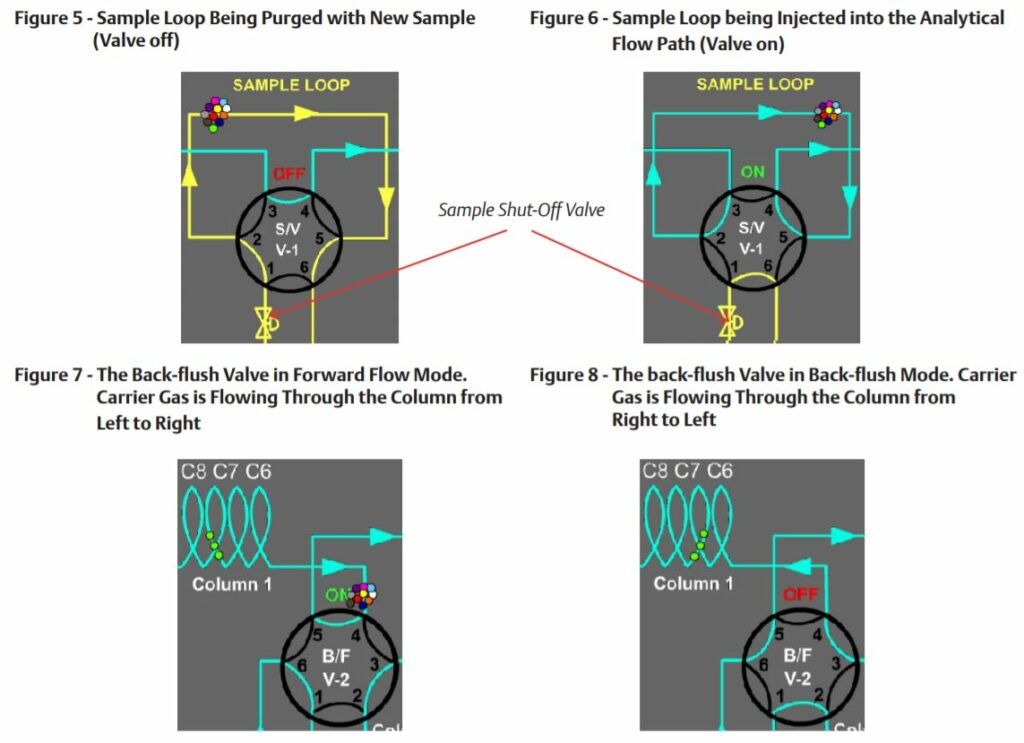
The sample valve is used to inject a repeatable volume of sample into the analytical flow path at the beginning of the analysis cycle. Before the sample injection, the sample loop is flushed with new sample (refer to Figure 5). To ensure the physical amount of sample is the repeatable from one analysis cycle to the next and is not affected by sample pressure, the sample flow is stopped for a short time before the sample injection using a sample shut-off valve, and the sample loop is allowed to equalize to atmospheric pressure via the sample vent.
To inject the sample into the analytical flow path, the sample injection valve is actuated and the carrier gas is switched so as to push (or “carry”) the sample out of the sample loop and into the first column (refer to Figure 6).
Two very common column switching techniques are used with multiple column gas chromatographs: the back-flush and the dual- column arrangement. The back-flush arrangement (refer to Figure 7) is used to separate the individually measured components from the heavy components which are sent to the detector as a single peak (such as the C6+ peak). The sample is pushed through the back-flush column until the last of the lighter components has left the back- flush valve, but before any of the back-flushed componentshave left the column. The back-flush valve is then actuated and the carrier gas direction through the column is reversed so that the heavy components (which have been partially separated in the column) are recombined into a single peak (refer to Figure 8).
GC Column Working Principle
The second column switching technique is the dual-column technique in which the analysis valve switches the flow path between two columns. Initially, lighter components are partially separated by a previous column and are directed into a column (column three in Figure 4) designed to fully separate the individual components. When all of the lighter components have entered the column, but before the first of the fully separated components have entered the valve, the dual-column valve is switched (refer to Figure 9) and the lighter components are trapped.The flow of carrier gas and sample components is now switched to the second column (the restrictor column marked by a 1 in a triangle in Figure 4), which could either conduct further separation of the components, or only act as a restriction to flow (as shown). The flow rate through the analytical flow path is dependent on the supply pressure of the carrier gas (which is constant) and the restriction to flow through the entire analytical flow path. Therefore, the second column on the dual- column arrangement must have the same restriction to flow as the column that has been switched out of the analytical flow path.Once all of the components being separated by the previous column have passed through the restrictor column and gone through to the detector, the dual-column valve is then actuated and the carrier gas flow is routed back through the original column to separate the lighter components and then carry them to the detector.
Detectors :
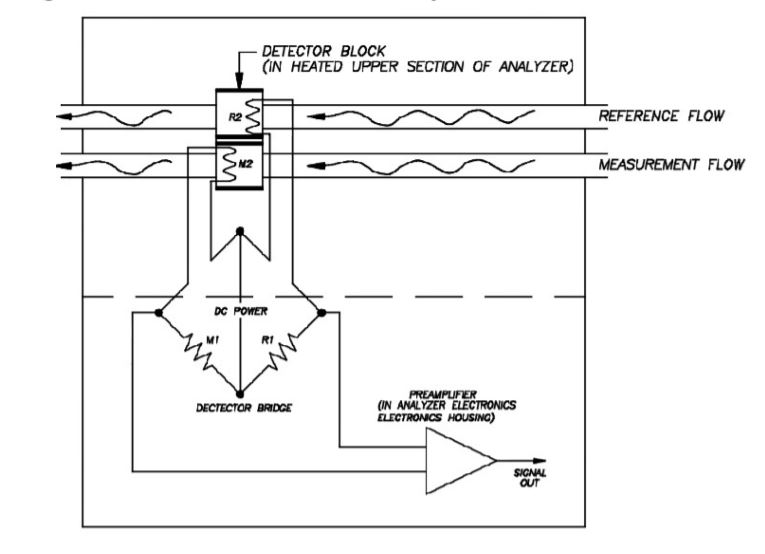
After the components have been separated by the chromatograph columns, they then pass over the detector. Several types of detectors are available for gas chromatographs, including flame ionization detectors (for ppm-level hydrocarbons) and flame photometric detectors (for ppb- to ppm-level sulphur detection), but the most common detector used for most hydrocarbon gas measurements is the thermal conductivity detector (TCD) (refer to Figure 10).The TCD can measure components from the low ppm levels up to 100 % concentration. The TCD uses two thermistors that will reduce in resistance as their temperature rises. The thermistors are connected on either side of a Wheatstone bridge with a constant current power supply. As the carrier gas passes over the thermistors, it removes heat from the thermistor bead, dependent on the carrier gas’s thermal conductivity. Helium is a commonly used carrier gas because it has a very large thermal conductivity, and, therefore, will reduce the temperature of the thermistors bead considerably.
GC Detector Working Principle
On the reference side of the detector, only pure carrier gas will pass over the thermistor bead, so the temperature and resistance will remain relatively constant.On the measure side of the detector, the carrier gas and each component in series of elution from the columns passes over the thermistor bead, removing heat from the bead dependent on the thermal conductivity. When there is only carrier gas passing over the detector bead, the temperature of the bead will be similar to the reference detector (any difference is compensated for using the bridge balance).
However, the gas components will have different thermal conductivities than the carrier gas. As the component flows across the thermistor bead, less heat is removed from the bead, so the temperature of the thermistor increases, reducing the resistance. This change in resistance imbalances the electrical bridge and results in a milli-voltage output. The amount of difference and, therefore, the output signal is dependent on the thermal conductivity and the concentration of the component.The detector output will then be amplified and passed to the gas chromatograph controller for processing.
Gas Chromatograph Controller : The controller may be remote from the oven or integral, depending on the design and application, and performs several functions, including:■ Control the valve timing and oven temperature■ Control stream selection■ Store and analyze the detector output■ Calculate composition from the detector output■ Calculate physical properties from the composition (e.g. BTU, Specific Gravity)■ Communicate to supervisory systems such as flow computers orSCADA systems.The unique function of the controller is the analysis of the detector output. The detector output is graphically displayed on a chromatogram (refer to below Figure ) that will typically also show the valve timing, the expected retention times of each of the components to be measured, the composition and calculation results, and raw detector data.Each measured component in the sample gas will have a peak (refer to below Formula Figure), which is the change in detector output because of the component passing over the measure detector. The controller determines which component the peak represents by the retention time, the time from the beginning of the analysis cycle to when the highest point of the peak. From one analysis cycle to the next, the retention time should not change significantly, so the expected retention time for each component and a detection window (in ± seconds) is configured in the controller.
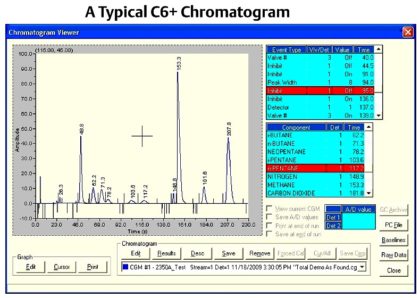
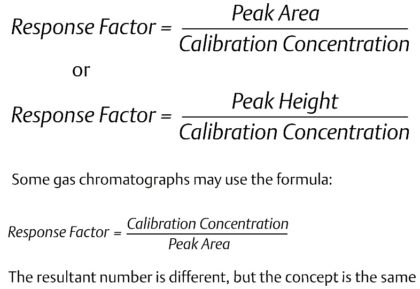
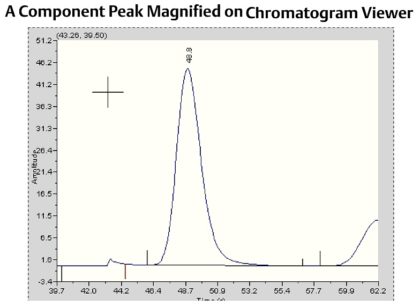
The concentration of the component in Gas Chromatography is proportional to the peak area, and the peak height. To calculate the peak area, the controller detects the start and end of the peak by analyzing the change in slope of the detector trace. On the chromatogram shown, the detected peak start is indicated by a large line above the baseline at around 46 seconds, and the detected peak end is indicated by the small line above the baseline at around 56 seconds. The controller will integrate the area under the detector trace and use this value to calculate a concentration value. Peak height is also calculated from the baseline to the highest point of the peak, and may be used as an alternative method to calculating the concentration.Before a concentration can be calculated, the gas chromatograph needs to be calibrated to determine the response factors for each individual component. The response factor is effectively the calibration factor for each individual component. To calculate the response factors, the gas chromatograph analyzes the calibration gas that has known concentrations for each of the measured components using one of the following formula:The gas chromatograph is calibrated at the factory, and will then be calibrated onsite on a regular basis using a certified calibration gas mixture. Gas chromatographs used for custody transfer applications are typically calibrated once a day (to validate the unit is working correctly, rather than to correct for drift), but many gas chromatographs can operate accurately between calibrations for several months in less critical plant applications.
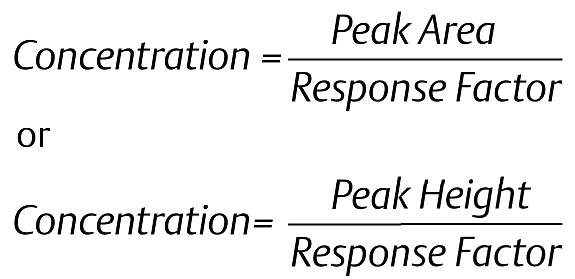
When performing an analysis one of the following formulas is used to calculate the concentration of each component, dependent on the peak detection method (area or height):
Many gas chromatographs perform a complete analysis for all of the major components expected in the sample, such as the natural gas “BTU” measurement. Theoretically, the sum of the concentrations of all of the measured components should equal 100 %. However, in reality the result rarely does equal 100 %. The cause of this “error” can be the result of1) the uncertainty of the detector measurement,2) slight changes in the sample loop pressure (because of atmospheric pressure changes) resulting in changes in the amount of gas sample injected, or3) measurement error. The total of the components as measured is referred to as the un-normalized total and is commonly expected to be between 98 % and 100 % for a correctly functioning gas chromatograph.As the analysis from the gas chromatograph is often used for calculating physical properties using calculation methods that presume the concentration total is 100 %, the analysis results are mathematically adjusted, or normalized, so that the sum of the components is exactly 100 %. Once the controller has calculated the full analysis, other physical property calculations may be performed.This may include:■ BTU/scf (according to GPA 2172-09 and GPA 2145-09)■ Standard Compressibility and Specific Gravity (AGA report No. 8–1994)■ Heating Value (ISO 6976)■ Hydrocarbon Dew Point
Chromatogram Viewer
The analysis results, analyzer status and calculation results are typically communicated to a supervisory system (such as a flow computer, RTU, or SCADA system) using a modbus serial link. Most chromatographs are capable of providing a 4–20 mA output; however, because of the large number of values that a chromatograph produces (up to 16 component concentrations, several physical property calculations, and analyzer status signals) using discrete signals is impractical. The serial links are commonly RS-232, RS-485, RS-422 or TCP/IP signals. Recent developments have seen the use of FOUNDATION™ Fieldbus communications to DCS-style control system.
Article Source : Emerson