Haber Process (Ammonia Manufacturing)
In industry Ammonia ia manufactured on a large scale by using Haber -Bosch Process.
Raw Material :
- Nitrogen (From Air)
- Hydrogen (Derived from Natural Gas : Methane)
Physical Characteristic
- Other Name : Hydrogen nitride or Trihydrogen nitride or Nitrogen trihydride
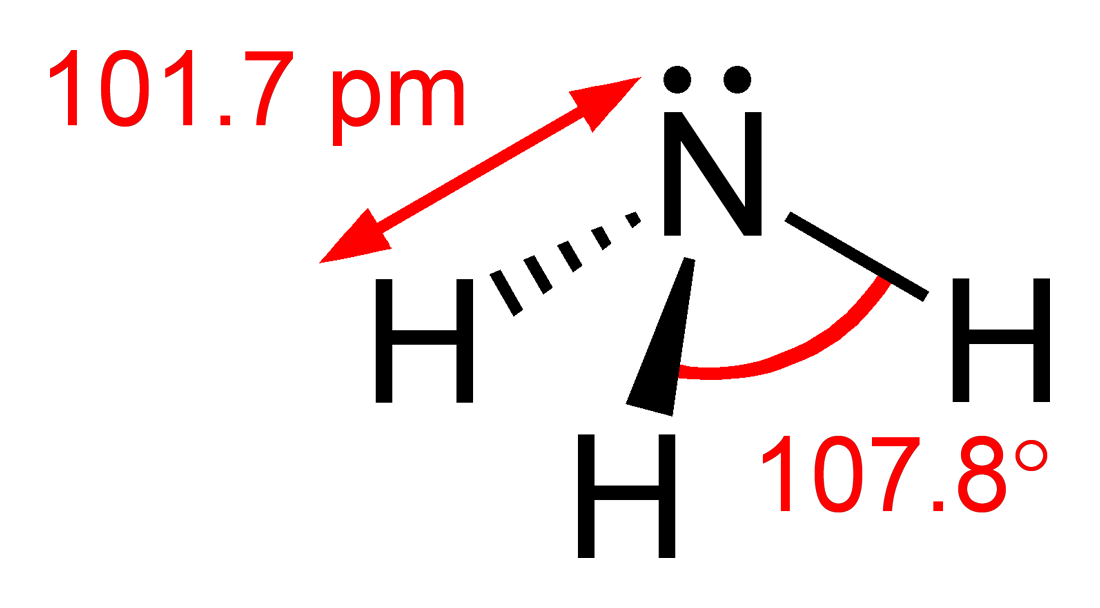
- Molar Mass : 17.031 g/mol
- Appearance : Colourless

- Odour : Strong Pungent Smell
- Solubility in water : 47% w/w (0 °C) ; 31% w/w (25 °C) ;18% w/w (50 °C)
- Solubility : Soluble in chloroform, ether, ethanol, methanol
- Molecular Structure

In the Haber Bosch Process nitrogen reacts with the hydrogen (derived mainly from natural gas : methane) to form ammonia. This reaction is highly reversible exothermic in nature.
Operating Pressure : 200 bar
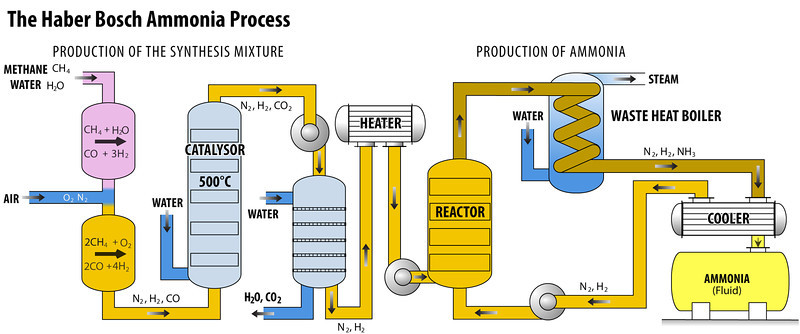
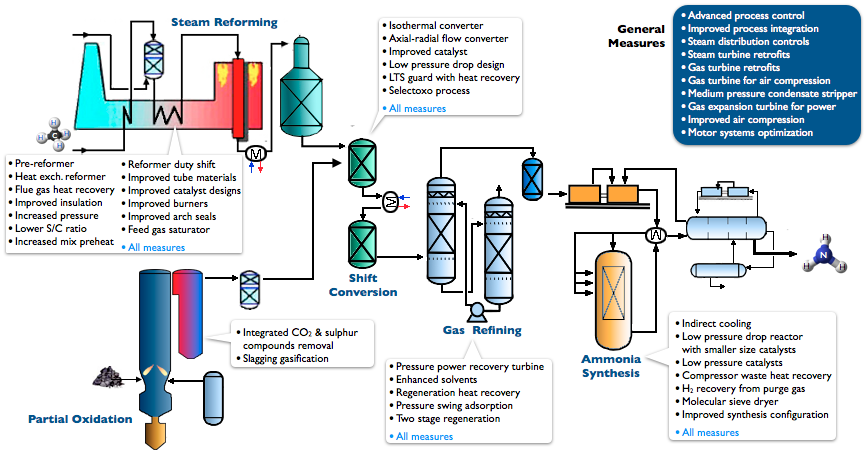
One of the main Byproduct of this is Carbon Dioxide Formation. Ammonia is the main building block for ammonium nitrate fertilizer.
Applications :
- Hydrazine and Hydroxylamine are the main derivatives of Ammonia.
- Fertilizer (90% of ammonia is used in fertilizer industry).
ImageSource : wikipedia