The Three Laws of Thermodynamics
The term “thermodynamics” comes from two root words: “thermo,” meaning heat, and “dynamic,” meaning power. Thus, the Laws of Thermodynamics are the Laws of “Heat Power.”
Learning Objective
- Discuss the three laws of thermodynamics.
Key Points
-
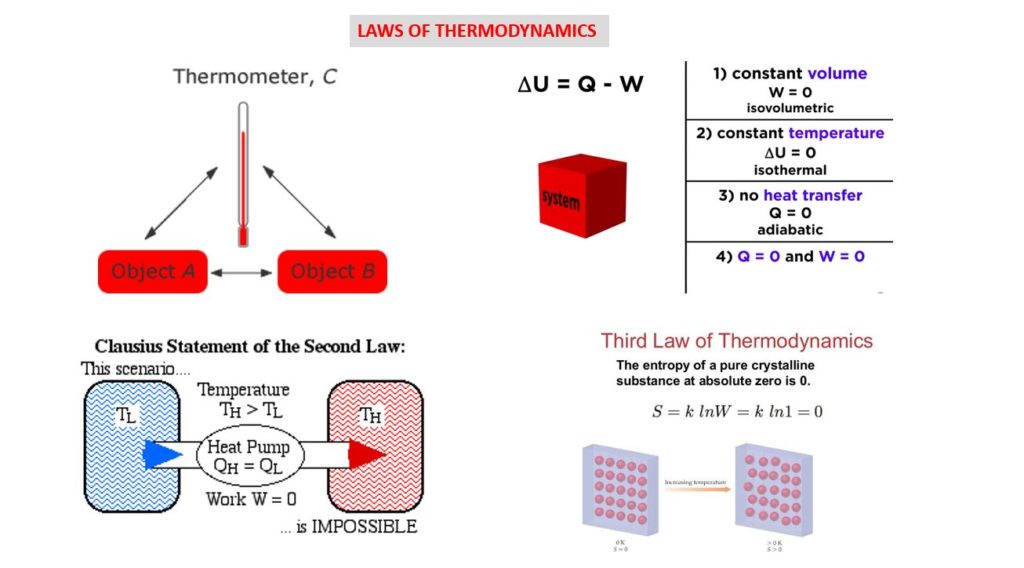
- The first law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic systems. It states that energy cannot be created or destroyed in an isolated system. The quantity of matter/energy remains the same. It can change from solid to liquid to gas to plasma and back again, but the total amount of matter/energy in the universe remains constant.
- The second law of thermodynamics states that the entropy of any isolated system always increases. The 2nd law of thermodynamics is commonly known as the Law of Increased Entropy.
- The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches “absolute zero“. This is the bottom point on the Kelvin temperature scale. The Kelvin scale is absolute, meaning 0° Kelvin is mathematically the lowest possible temperature in the universe. This corresponds to about -273.15° Celsius, or -459.7 Fahrenheit.
Terms
- Absolute zero is the lowest temperature that is theoretically possible.
- Entropy : A thermodynamic property that is the measure of a system’s thermal energy per unit of temperature that is unavailable for doing useful work.
System or Surroundings
In order to avoid confusion, scientists discuss thermodynamic values in reference to a system and its surroundings. Everything that is not a part of the system constitutes its surroundings. The system and surroundings are separated by a boundary. For example, if the system is one mole of a gas in a container, then the boundary is simply the inner wall of the container itself. Everything outside of the boundary is considered the surroundings, which would include the container itself.
The boundary must be clearly defined, so one can clearly say whether a given part of the world is in the system or in the surroundings. If matter is not able to pass across the boundary, then the system is said to be closed; otherwise, it is open. A closed system may still exchange energy with the surroundings unless the system is an isolated one, in which case neither matter nor energy can pass across the boundary.
A Thermodynamic System : A diagram of a thermodynamic system
The First Law of Thermodynamics
The first law of thermodynamics, also known as Law of Conservation of Energy, states that energy can neither be created nor be destroyed; energy can only be transferred or changed from one form to another. For example, turning on a light would seem to produce energy; however, it is electrical energy that is converted.
A way of expressing the first law of thermodynamics is that any change in the internal energy (∆E) of a system is given by the sum of the heat (q) that flows across its boundaries and the work (w) done on the system by the surroundings:
This law says that there are two kinds of processes, heat and work, that can lead to a change in the internal energy of a system. Since both heat and work can be measured and quantified, this is the same as saying that any change in the energy of a system must result in a corresponding change in the energy of the surroundings outside the system. In other words, energy cannot be created or destroyed.
If heat flows into a system or the surroundings do work on it, the internal energy increases and the sign of q and w are positive. Conversely, heat flow out of the system or work done by the system (on the surroundings) will be at the expense of the internal energy, and q and w will therefore be negative.
The Second Law of Thermodynamics
The second law of thermodynamics says that the entropy of any isolated system always increases. Isolated systems spontaneously evolve towards thermal equilibrium—the state of maximum entropy of the system. More simply put: the entropy of the universe (the ultimate isolated system) only increases and never decreases.
A simple way to think of the second law of thermodynamics is that a room, if not cleaned and tidied, will invariably become more messy and disorderly with time – regardless of how careful one is to keep it clean. When the room is cleaned, its entropy decreases, but the effort to clean it has resulted in an increase in entropy outside the room that exceeds the entropy lost.
The Third Law of Thermodynamics
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has. Specifically, the entropy of a pure crystalline substance (perfect order) at absolute zero temperature is zero. This statement holds true if the perfect crystal has only one state with minimum energy.
ImageCredit : Lumen Learning