Sulphuric Acid, also known as Vitriol is composed of elemental sulphur, Hydrogen and Oxygen.
The molecular formula is :
Physical Characteristics : Colourless; Odorless; Soluble in water and is highly exothermic in nature. Considered as Strong Acid.
Chemical Characteristic : Highly corrosive in nature, Hygroscopic, Produces severe chemical and thermal burns when in contact.
Aslo Read This :
Vinyl Chloride from Ethylene
Urea Production and Manufacturing
Cement Manufacturing Process
Industrial Sulphuric Acid Production
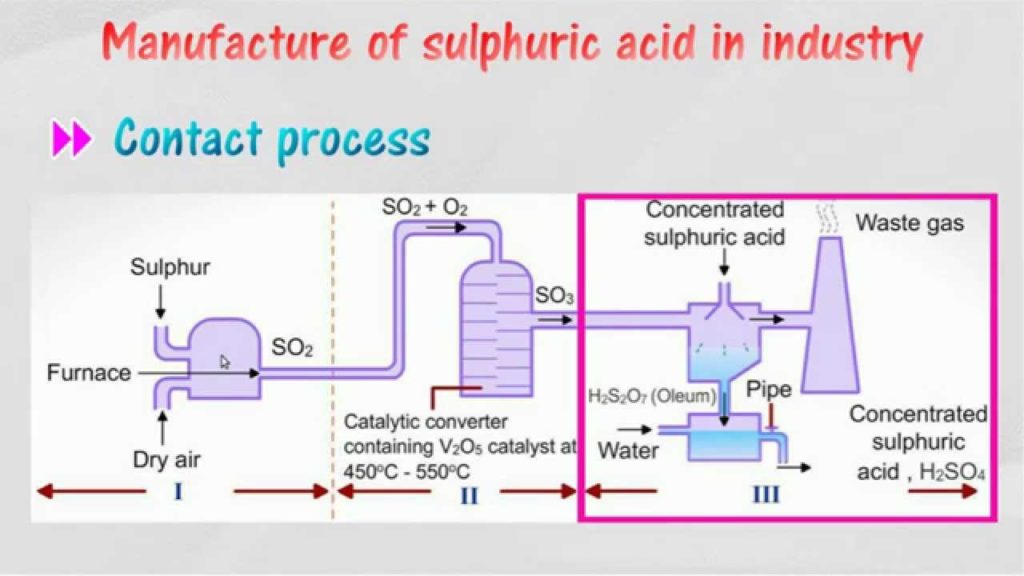
In industry sulphuric acid is mainly produced by
- Contact Process :
- Wet Sulphuric Acid Process
- Lead Chamber Process : Produces Dilute Sulphuric Acid (67-78%
Chemical Reactions Involved :
Step 1 : Sulphur is burned to produced Sulphur Oxide
Step 2 : Then Sulphur Oxide is oxidized to sulfur trioxide using oxygen in the presence of a vanadium(V) oxide catalyst
The sulfur trioxide is absorbed into 97–98%
Sulphuric Acid Production Process Flow Sheet
Industrial Sulphuric Acid Plant
Sulphuric Acid Applications
- Paint & Pigments
- Pharma & Bio- Pharmaceuticals Industries
- Fertilizer Manufacturing
- Chemical Synthesis
- Uses in Oil and Waste water processing Industries
- Manufacturing Cleaning Agents
- Batteries
- Plastic Industry
ImageSource : Wikipedia; askIITians; DuPont;
ArticleSource : Wikipedia