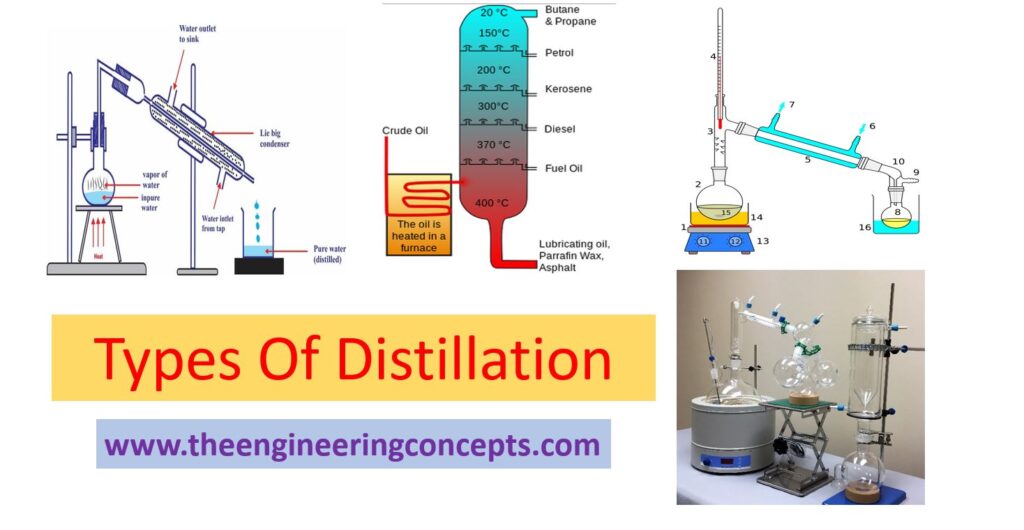
Distillation
The term Distillation is no new word to any person mainly for a chemical engineer. To put in simple terms, Distillation is a procedure that separates a mixture of liquids having different boiling points or Distillation is the process of purification of compounds based on the relative volatility of the mixture’s component. Distillation process is basically used either for increasing the concentration of a particular component in the mixture Or, to obtain almost (99.9%) specific pure component from the mixture. The different types of distillation include Simple, Fractional, Steam, Vacuum, and Short path distillation. Other types include Air-sensitive vacuum distillation, Zone distillation. The working principle of distillation is to heat a mixture at a specific temperature, collect the hot vapors, and condense to separate the component substance. In detail, a highly volatile compound is separated from a less-volatile or non-volatile compound by using distillation.
Article By : KAASHYAP P
Simple Distillation
A liquid mixture is boiled and the resulting vapors passes through the apparatus until they reaches the condenser where they are cooled and re-liquefy. Liquids are separated based upon the differences in their boiling point.
Figure1: Simple Distillation
Fractional Distillation
Fractional distillation is the process of separating a liquid mixture into fractions, Considering different component has different boiling points at different temperature. The vapors of different component of the liquid mixture at different temperature are vaporized followed by condensation and are get collected in purified liquid form. An example about fractional distillation is of crude oil, As crude oil is heated the vapors start rising in the column, heavier components (Fuel Oil, Bitumen, Asphalt etc.) are pulled out in the lower of the column and lighter components as: Gasoline, Diesel and Kerosene rises higher to the column and are pulled out from the top of the column.
Figure 2: Fractional Distillation
Steam Distillation
Steam distillation is carried out for purifying temperature-sensitive materials, such as natural aromatic compounds. In steam distillation, dry steam is passed through the plant material, mixture is heated and the components are get vaporized below their decomposition temperature. These vapors undergo condensation and are get collected into the respective receivers. Steam distillation implements low-pressure steam, this replaces the volatile compounds from the intact plant material. It also allows to control the temperature and amount of steam. In case of extraction of essential oils is carried out by using steam distillation technique. This technique is used in the isolation of antioxidant and antimicrobial compounds from herbs & species.
Vacuum distillation
In case of vacuum distillation, distillation process is performed under reduced pressure i.e. lower than the ambient pressure. Vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (Tb>150 oC) in order to distill the compound without significant decomposition or thermal degradation. The application of vacuum distillation is mainly found in petroleum refining where the residue from the bottoms of the Atmospheric Distillation Unit (ADU) is fed to the Vacuum Distillation Unit (VDU) and are operated below atmospheric pressure (vacuum pressure). The vacuum distillation unit is classified into wet and dry design (the steam is not introduced during the operation). The wet design towers are preferred in the refineries.
Figure 4: Vacuum Distillation
Short Path Distillation
Short path distillation is a technique in which distillate traverses very compact path (Or, short path); is suitable for laboratory applications operated normally at reduced pressure. A liquid solution or emulsion is suspended in an evaporating flask, connected to via a condenser unit. The evaporating flask is positioned in a heating mantle that slowly increases the temperature of the solution to pre-defined boiling points. The advantage is that the heating temperature can be considerably lower (at reduced pressure) than the boiling point of the liquid at standard pressure, and the distillate only has to travel a short distance before condensing. Distillates are collected accordingly in separate respective flasks.
Here in start heating of the flask is done at lowest boiling point of the mixture’s component. gradually vapors are condensed and get collected in respective flask. Now the heating value increases and set at higher boiling point of the mixture’s component. Thus respective component vaporizes followed by condensation and get collected in another flask. And the process repeated until no further separation is required.
Note : This technique is suitable for those compounds which are highly unstable at high temperatures.
Zone distillation
Zone distillation involves the partial melting of refined material in moving liquid zone and condensation of vapor in the solid phase at condensate, pulling in cold area. Zone heater is used to carry out the process. When zone heater is moving from the top to the bottom of the container then solid condensate with irregular impurity distribution is forming. Then most pure part of the condensate may be extracted as product. The process may be iterated many times.
Figure 6 : Zone Distillation
Air Sensitive Vacuum Distillation
Some compounds having high boiling point but they are air sensitive in nature. For air sensitive high boiling compounds, a simplified distillation is modified with inert gas as a replacement to the steam. A Perkin triangle is a specialized apparatus for the distillation of air-sensitive materials.
Figure 7: Air Sensitive Vacuum Distillation
The Perkin triangle uses a series of glass or Teflon taps to allow fractions to be isolated from the rest of the still, without the main body of the distillation being removed from either the vacuum or heat source, and the reflux continues.
References:
Wikipedia; Sciencing; Chemistry Views; Biocyclopedia
ImageSource : ResearchGate; Energy Education; Steemit; Tnlab